Melting splitting
Methane is a very good friend of carbon dioxide, both of them are greenhouse gases and starting points of C1 chemistry. They are unwilling to get separate since carbon dioxide is the most thermodynamically stable resultant from conversion of methane. Their friendship causes huge emissions of carbon dioxide from industrial methane steam reforming to produce hydrogen.
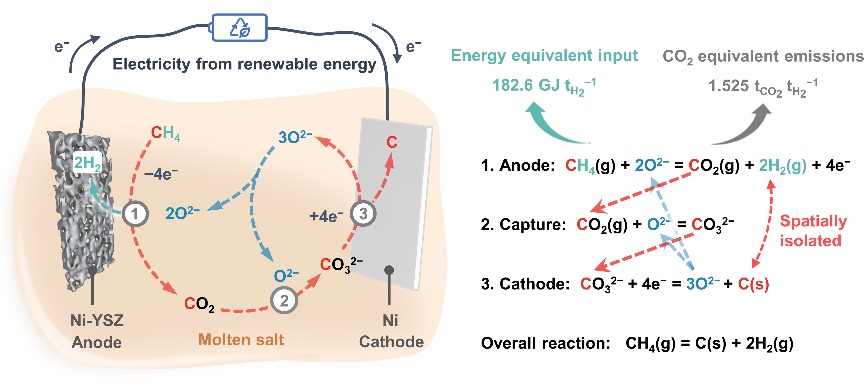
We recently demonstrate an electrochemical splitting of methane in molten salts to produce hydrogen in an emissions-free, water-free, coke-free and CO-free manner. Methane is electrochemically oxidized into hydrogen and carbon dioxide in Ni anode. The generated carbon dioxide is in situ captured by the melts and then transforms into solid carbon in Ni cathode. The molten salt serves as a melting ninja to split methane into anodic hydrogen and cathodic carbon, offering an efficient and green production of hydrogen. The above results are now accepted for publication in Angew. Chem. Int. Ed. as a VIP.
Many congratulations to Zeyu. A good start of 2021.