Calcium replaces Lithium
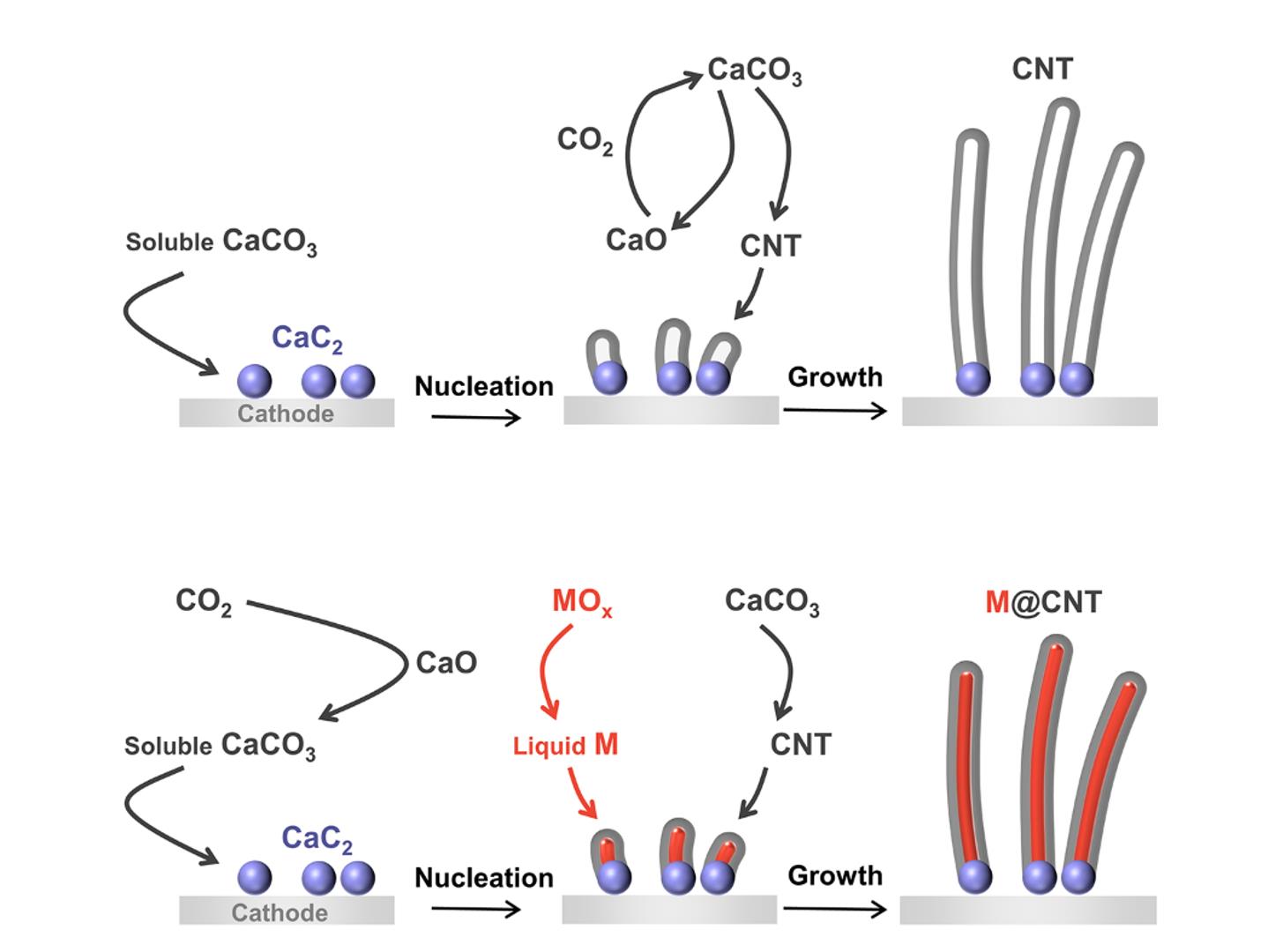
The large-scale application of capture and electrochemical reduction of CO2 is limited by the high price of lithium based molten salt and the low value of cathodic products. Electrochemical reduction of carbon dioxide in affordable Ca-based molten salt is demonstrated as an efficient preparation of carbon nanotube (CNT) and metal-confining CNT (M@CNT) by a CaC2-mediated mechanism in our recent study. Anodic CO2 is captured in situ from the graphite anode to generate CaCO3 by adding CaO, and the current efficiency of O2 evolution in graphite anode is increased. The whole process inputs CO2 and outputs CNT and O2, similar to photosynthesis. The current efficiency and energy efficiency of molten salt integrated artificial photosynthesis are 85.1% and 44.8%. Sn@CNT shows excellent lithium storage performance and interesting application for nanothermometer. The above results are now accepted for publication in Angew. Chem. Int. Ed. (DOI: 10.1002/anie.202306877).