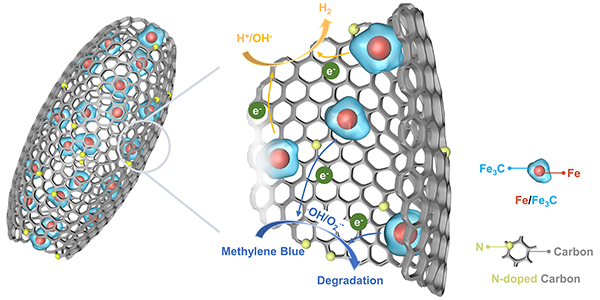
Interaction between iron and nonmetals is a classic topic in industrial iron/steel making and catalysis, in which the modulation of linkage and distribution of iron and nonmetals is a key-enabling factor to develop advanced Fe-based materials. We recently demonstrate a thermoelectrochemical coupling in molten salts to confine microstructure of Fe-N-C. The hybrid of Fe/Fe3C nanoparticles strongly encapsulated in a nitrogen-doped carbon (N-C) hollow shell is prepared through a thermoelectrochemical treatment of Fe2O3@polydopamine (Fe2O3@PDA) in molten NaCl-CaCl2 at 600 oC. The coupling of thermal pyrolysis of PDA and electrochemical reduction of Fe2O3 contributes to generating Fe/Fe3C species at a relatively low temperature, ensuring structure integrity and in situ N-doping for the hybrid to achieve well-defined hollow structure and abundant active sites of Fe-N and C-N species. The hollow carbon skeleton guarantees efficient mass transfer and maximum exposure of active sites. Consequently, the Fe/Fe3C@N-C exhibits appealing activity for the electrocatalytic hydrogen evolution and Fenton-like reaction, and importantly, can be readily separated for reuse by a magnetic field. The above results are now accepted for publication in J. Mater. Chem. A (J. Mater. Chem. A, 2020, 8, 4800-4806).
Author: Prof. Xiao