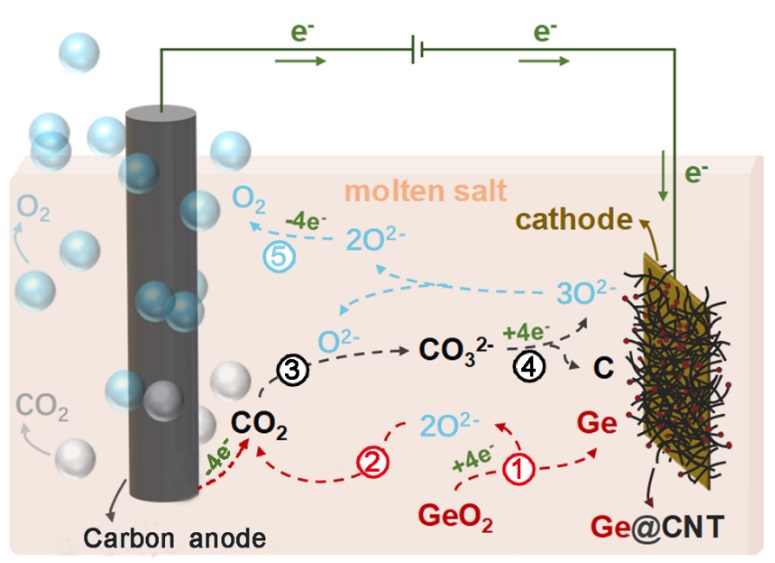
Fate of O2- in molten salts plays an important role in electrochemical reduction of oxide ores and electrochemical fixation of CO2. Our recent investigation shows that such two processes can elaborately be connected in serial in molten salts for advanced metallurgy and in-situ CO2 fixation. The delicately designed molten salt electrolyzer consists of molten NaCl-CaCl2-CaO as electrolyte, soluble GeO2 as Ge feedstock, conducting substrates as cathode and carbon as anode, which has a similar configuration to the Hall-Heroult cell for industrial Aluminum production. An unprecedented cathode-anode synergy is verified as co-electrolysis of soluble GeO2 and in-situ generated CO2 at carbon anode to cathodic Ge nanoparticles encapsulated in carbon nanotubes (Ge@CNTs), contributing to enhanced oxygen evolution at carbon anode, reduced CO2 emissions, and upgraded conversion of carbon bulks to CNTs. The above results are now accepted for publication in Science Advances (DOI: 10.1126/sciadv.aay9278).
Author: Prof. Xiao